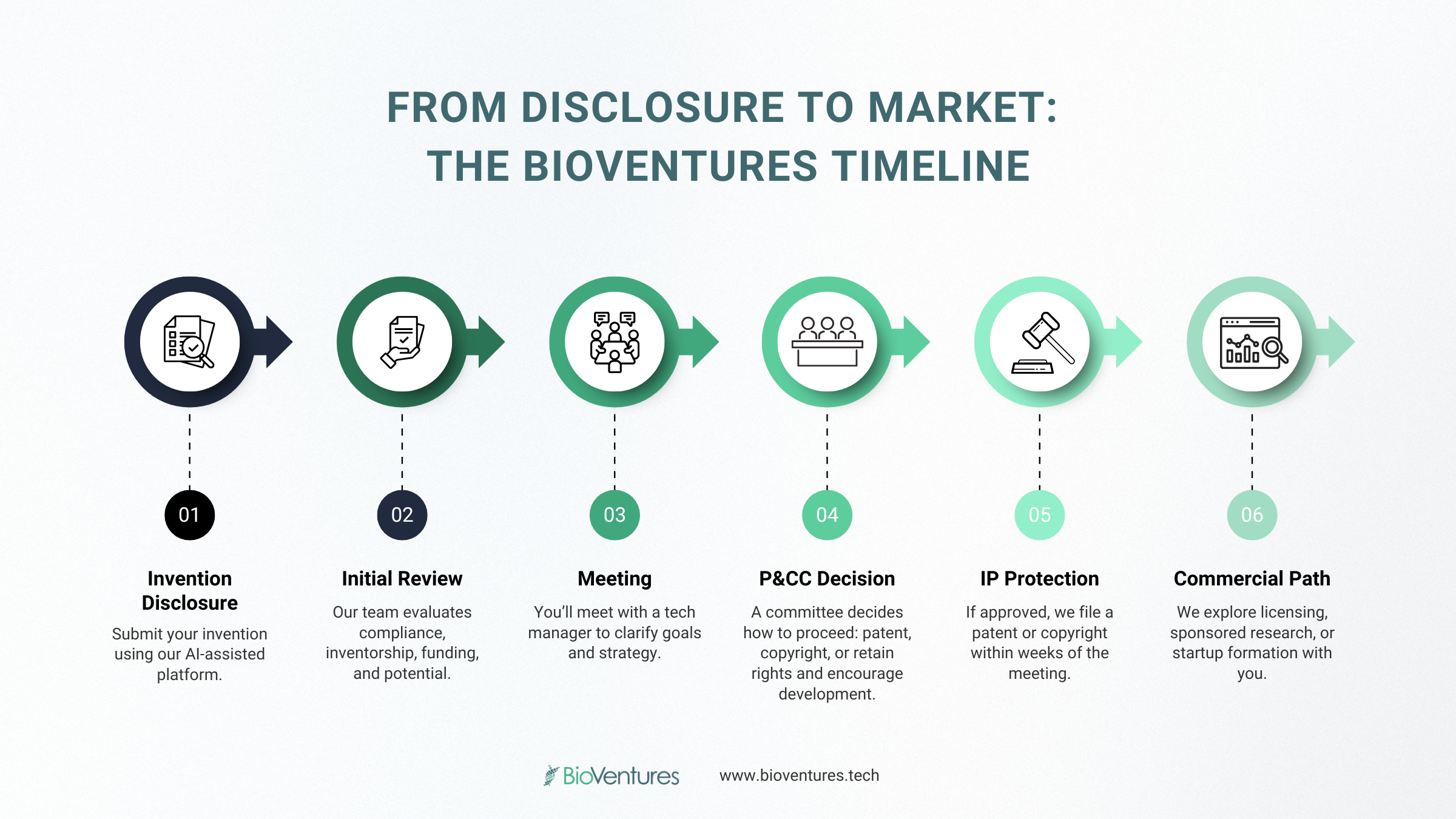
UAMS Showcase of Medical Discoveries Highlights BioVentures-Supported Innovation
The latest UAMS Showcase of Medical Discoveries, held on October 22 at the Jackson T. Stephens Spine & Neurosciences Institute, celebrated the spirit of innovation and entrepreneurship driving research at UAMS. The event featured projects supported by BioVentures LLC, UAMS’ technology transfer office and business incubator, underscoring how discoveries in the lab are evolving into real-world health solutions and startups across Arkansas. Eric Peterson, Ph.D., President of BioVentures, opened the event by highlighting the courage it takes for researchers to move their discoveries toward commercialization, noting that “commercialization is a pathway to getting biomedical inventions to the bedside.” Attendees explored a diverse range of innovations, from new cancer immunotherapies and diagnostic assays to medical devices, AI-driven imaging tools, and novel drug candidates. Projects included the Smart Tracheal Collar, a reusable monitoring device for pediatric patients with tracheostomies, and StaphGuard Biofilm, a first-in-class small molecule developed by VanGuard Biofilm LLC to prevent implant infections. Daniel Voth, Ph.D., UAMS Vice Chancellor for Research & Innovation, emphasized the critical role of BioVentures in helping UAMS investigators protect intellectual property, form startups, and connect with industry partners and investors. The event drew a strong crowd of faculty, researchers, and entrepreneurs, reflecting the growing momentum of commercialization efforts across the UAMS campus. Each project showcased the power of collaboration between science and business to advance healthcare and strengthen Arkansas’s biomedical economy. A full gallery of photos from the event is available below.