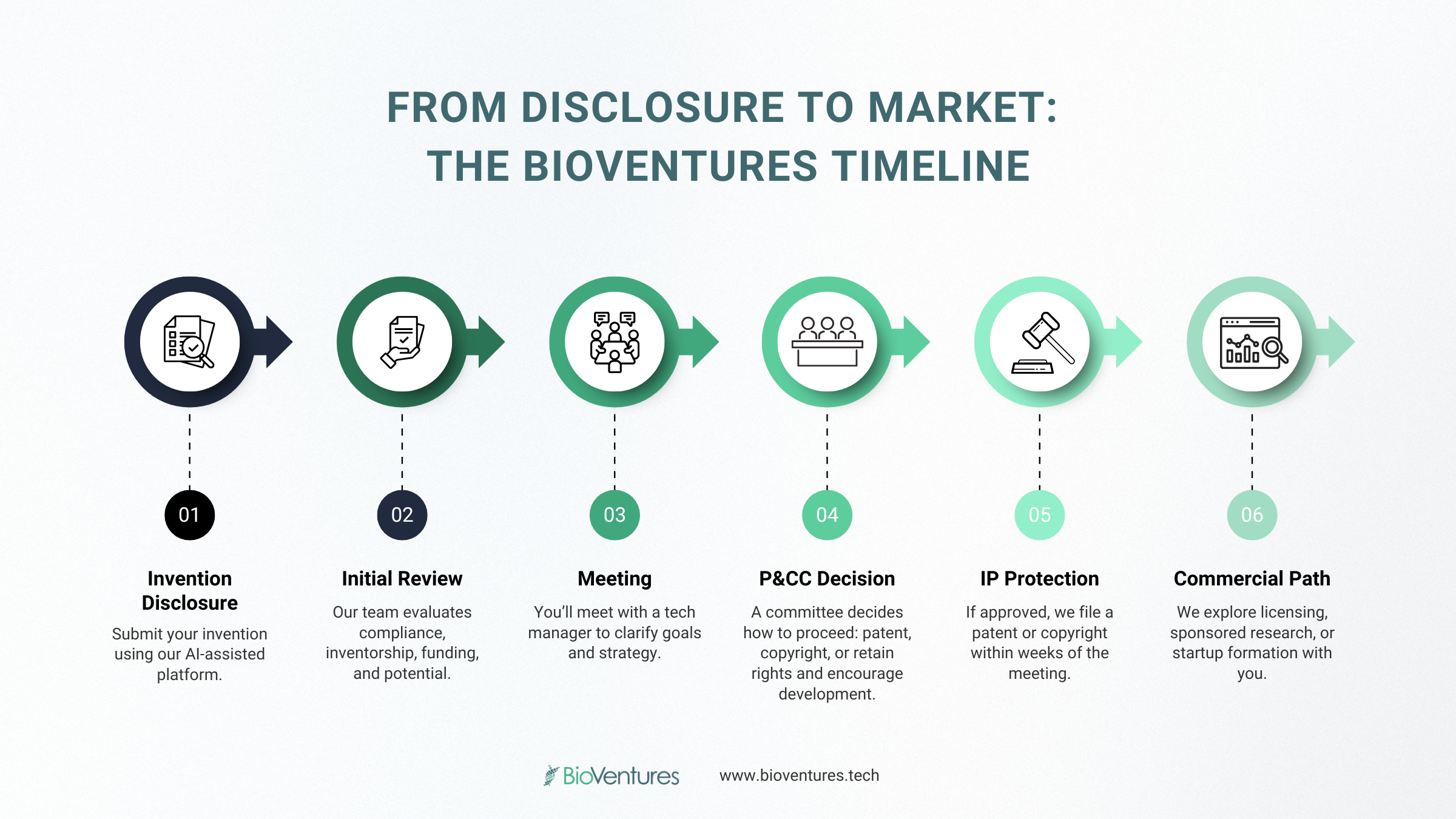
Curious how an idea becomes a protected invention at UAMS? At BioVentures, we guide innovators through every step, from disclosure to patent decisions and market strategy. Here’s how the process works, what to expect, and how we support you along the way. 1) Submit Your Invention Disclosure The first step is fully and adequately disclosing your invention to BioVentures by submitting an invention disclosure. Even if you’ve already reached out to schedule a meeting, we require a disclosure to initiate the process. It allows us to review the technology in advance, and gather essential information on compliance, funding, and inventorship. We’ve recently adopted a new AI-powered disclosure platform to simplify the process. You can upload relevant documents—papers, figures, posters, presentations—and the system will generate a draft disclosure for you to review and edit before submission: 👉 Submit your disclosure The form captures a complete overview of your invention, its potential applications, and any existing data or prototypes. Once submitted, BioVentures typically reviews the disclosure within 1–2 weeks before scheduling a meeting with you. Typical timing: BioVentures review in 1–2 weeks. 2) Meeting with the Technology Manager We meet with the inventor to clarify the technology, confirm federal compliance needs, capture inventor and funding information, and discuss long‑term goals and commercialization paths. Typical timing: Scheduled within 1–2 weeks after disclosure review. 3) P&CC Decision (Patent & Copyright Committee) The inventor presents at the P&CC. This committee reviews the presented technology and makes a determination on how the technology should be protected. Outcomes can include: Typical timing: P&CC is usually scheduled 1–2 months after acceptance of the disclosure. 4) Protection Strategy Based on P&CC Outcome The legal protection route follows the P&CC decision. If a provisional patent filing is recommended, we move quickly to secure a priority date and a 12‑month window to strengthen data and assess market potential. This is because in the United States is a first to file country which means that the right to a patent generally belongs to whichever inventor files an application with the USPTO first, not to whoever first conceives the idea. As such, protecting your invention in a timely manner is important to us. Typical timing: Provisional filing is typically completed within a few weeks of the P&CC meeting. 5) Marketing and Commercial Pathways If the inventor agrees, we begin targeted outreach. Options include: Why Early Disclosure Matters Submitting your invention disclosure early is essential. It helps preserve your patent rights before any public disclosure or publication, ensuring your ability to pursue protection. Early disclosure also allows BioVentures to align your IP strategy with funding sources and compliance requirements, and accelerates our ability to evaluate the market potential and identify relevant partners. This overview is for general information and does not constitute legal advice. Specific strategies and timelines may vary by technology. Have an invention to disclose? Submit your disclosure now!