
Immersive Distraction Therapy: A New Way to Reduce Surgical Anxiety
Newly granted patent: U.S. Patent No. 12,370,338 B2, “Immersive Distraction Therapy.

Newly granted patent: U.S. Patent No. 12,370,338 B2, “Immersive Distraction Therapy.

Join us on October 22, from 4–6 PM at the Jack Stephens Spine Institute (12th Floor) for the next UAMS Showcase of Medical Discoveries, focused on UAMS intellectual property and innovations. The event will feature technologies developed by UAMS researchers and supported through BioVentures, offering a look at discoveries with the potential to improve health outcomes and drive commercialization. Visit the official event page for more details.

We were excited to host the kickoff meeting for one of our newest startups, OncoRock Therapeutics, right here at BioVentures! OncoRock is a biotechnology startup dedicated to transforming academic discoveries into impactful cancer treatments. Their capital-efficient model focuses on acquiring promising university assets, de-risking them through preclinical studies and early-phase clinical trials, and then partnering or licensing to pharma for late-stage development. The company is advancing two first-in-class programs: The founding team of OncoRock brings together deep expertise across oncology, immunology, and commercialization. Led by Alan Tackett, PhD (CEO), Brian Koss, PhD (COO), Nancy Rusch, PhD (CSO), Laura Hutchins, MD (CMO), and Sonet Weed, MS (CFO), the group has collectively secured over $100M in research funding and combines decades of experience in cancer research, clinical trials, drug development, and biotech entrepreneurship. We’re thrilled to see their journey begin in our space and look forward to supporting their mission of bringing breakthrough therapies to patients with limited treatment options.

Do you have a passion for advancing scientific innovation through program leadership? BioVentures is looking for a Research Program Manager to help us build the future of biomedical entrepreneurship and translational research in Arkansas. This role is perfect for someone who thrives at the intersection of science, strategy, and operations. What you’ll do: • Lead and manage multi-year grants that support innovation, entrepreneurship, and commercialization • Coordinate educational programs that help researchers turn discoveries into real-world solutions • Collaborate with faculty, industry partners, funders, and leadership to move complex projects forward • Oversee budgets, reporting, compliance, and timelines across multiple initiatives Why join us? • Be part of a mission-driven team supporting health innovation at UAMS and beyond • Help grow Arkansas’s biomedical innovation ecosystem • Gain exposure to federal funding agencies, and state and national commercialization networks • Work in a collaborative environment that values initiative, creativity, and impact About you: • You have experience in biomedical research, grant management, or academic programs • You’re highly organized, proactive, and an excellent communicator • You bring a strong understanding of higher ed or biomedical research environments • Familiarity with entrepreneurship, tech transfer, or commercialization is a plus, but curiosity and willingness to learn are just as important Apply now: Research Program Manager – UAMS / BioVentures Know someone who’d be great in this role? Please share!

We’re proud to share that our Senior Licensing Associate, Megan Reed PhD, MBA has been selected as a 2025 recipient of the Susan Riley Keyes Memorial Fellowship from the AUTM Foundation. This highly competitive fellowship supports early-career technology transfer professionals through: • Mentorship from seasoned leaders in the field • Specialized training in invention evaluation, patenting, licensing, and commercialization • Full access to AUTM educational resources, including the Technology Transfer Practice Manual and webinar library • Registration, travel, and lodging for the AUTM Essentials Course, AUTM Annual Meeting, and regional meetings • Networking opportunities with a national cohort of fellows and industry experts Only three fellows were selected from a pool of 50 applicants, and Megan is one of them. This fellowship will deepen her expertise and broaden her professional network, further strengthening BioVentures’ ability to support UAMS innovators and drive the commercialization of life-changing biomedical technologies. Join us in congratulating Megan on LinkedIn!

Newly granted patent: U.S. Patent No. 12,268,674 B2, “Mi-2β Inhibitor as an Immunotherapy Agent” What’s the problem? Immune checkpoint therapies, such as anti-PD-1 antibodies (e.g. Keytruda, Opdivo), have transformed cancer treatment. Yet many patients either don’t respond at all or eventually develop resistance. This limits their long-term effectiveness, leaving a major gap in oncology care. What does this technology do? This patent covers novel small-molecule inhibitors that block the ATPase pocket of Mi-2β (CHD4), a chromatin remodeling protein identified as a key driver of PD-1 resistance. One lead compound, Z36-MP5, restores responsiveness to PD-1 therapy by: By directly targeting tumor-intrinsic resistance mechanisms, Mi-2β inhibitors open the door to a first-in-class therapeutic strategy to make immunotherapy work for more patients.

BioVentures will be present at the Translational Research Institute (TRI) Research Expo 2025, taking place Wednesday, September 10, from 4:00 to 6:00 p.m. CDT at the Reynolds Institute on Aging, First Floor. The Expo brings together more than 50 research services across UAMS, providing investigators with the opportunity to explore resources, build connections, and learn about available support for advancing their work. Visitors can stop by the BioVentures table to discuss invention disclosures, licensing, commercialization pathways, and opportunities to bring biomedical innovations to market. Don’t miss the chance to connect with colleagues, enjoy refreshments, and learn how BioVentures can support your research journey. 👉 Register here

Stefanie Kennon-McGill, Ph.D., Senior Program Manager at BioVentures LLC, will be the featured speaker at 2 p.m. Wednesday, August 27, for the Health Sciences Entrepreneurship Grand Rounds. The topic will be “Leveraging Commercialization to Further Your Impact.” Join us at the Winthrop P. Rockefeller Cancer Institute, 10th Floor, Betsy Blass Conference Room. The UAMS community and public are welcome to attend.
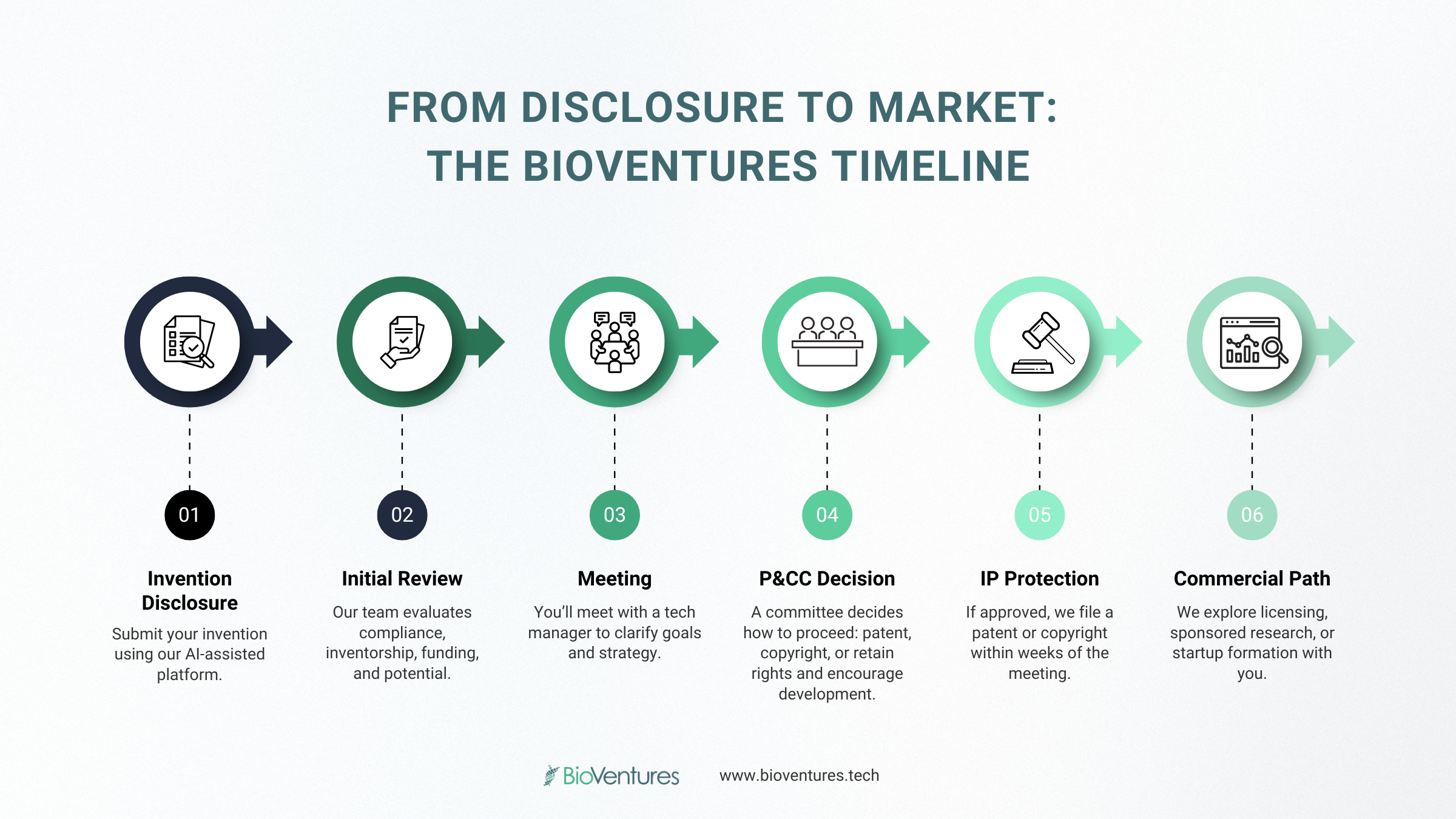
Curious how an idea becomes a protected invention at UAMS? At BioVentures, we guide innovators through every step, from disclosure to patent decisions and market strategy. Here’s how the process works, what to expect, and how we support you along the way. 1) Submit Your Invention Disclosure The first step is fully and adequately disclosing your invention to BioVentures by submitting an invention disclosure. Even if you’ve already reached out to schedule a meeting, we require a disclosure to initiate the process. It allows us to review the technology in advance, and gather essential information on compliance, funding, and inventorship. We’ve recently adopted a new AI-powered disclosure platform to simplify the process. You can upload relevant documents—papers, figures, posters, presentations—and the system will generate a draft disclosure for you to review and edit before submission: 👉 Submit your disclosure The form captures a complete overview of your invention, its potential applications, and any existing data or prototypes. Once submitted, BioVentures typically reviews the disclosure within 1–2 weeks before scheduling a meeting with you. Typical timing: BioVentures review in 1–2 weeks. 2) Meeting with the Technology Manager We meet with the inventor to clarify the technology, confirm federal compliance needs, capture inventor and funding information, and discuss long‑term goals and commercialization paths. Typical timing: Scheduled within 1–2 weeks after disclosure review. 3) P&CC Decision (Patent & Copyright Committee) The inventor presents at the P&CC. This committee reviews the presented technology and makes a determination on how the technology should be protected. Outcomes can include: Typical timing: P&CC is usually scheduled 1–2 months after acceptance of the disclosure. 4) Protection Strategy Based on P&CC Outcome The legal protection route follows the P&CC decision. If a provisional patent filing is recommended, we move quickly to secure a priority date and a 12‑month window to strengthen data and assess market potential. This is because in the United States is a first to file country which means that the right to a patent generally belongs to whichever inventor files an application with the USPTO first, not to whoever first conceives the idea. As such, protecting your invention in a timely manner is important to us. Typical timing: Provisional filing is typically completed within a few weeks of the P&CC meeting. 5) Marketing and Commercial Pathways If the inventor agrees, we begin targeted outreach. Options include: Why Early Disclosure Matters Submitting your invention disclosure early is essential. It helps preserve your patent rights before any public disclosure or publication, ensuring your ability to pursue protection. Early disclosure also allows BioVentures to align your IP strategy with funding sources and compliance requirements, and accelerates our ability to evaluate the market potential and identify relevant partners. This overview is for general information and does not constitute legal advice. Specific strategies and timelines may vary by technology. Have an invention to disclose? Submit your disclosure now!

BioVentures supported University of Arkansas for Medical Sciences (UAMS) first-year medical students this summer through the Partnership in Cancer Research (PCAR) program. Over eight weeks, participants worked in multidisciplinary teams to address real-world challenges in cancer prevention, diagnosis, treatment, or survivorship. The program culminated in entrepreneurial pitch presentations to a panel of judges. Three BioVentures team members served as mentors, advising students on refining problem statements, assessing market opportunities, and developing business models. Another team member participated as a judge, providing feedback from a commercialization perspective. The collaboration aimed to foster entrepreneurial thinking, highlight the role of innovation in cancer care, and encourage future clinician-innovators at UAMS. This initiative reflects BioVentures’ ongoing commitment to advancing healthcare entrepreneurship and translating research into real-world impact.